Software Validation Course
Software Validation Course - Software verification and validation (v&v) course by software excellence academy is designed to provide a knowledge base and practical skills for anyone interested in implementing or. Trusted certificationaccredited online coursescpd / ceu accreditation In just two weeks, you'll gain a comprehensive understanding of industry. Fda software validation guidelines training. Helping you become a certified csv. Courses include voiceovers, easy navigation, reading materials, case. Software testing and validation are vital because they ensure that the end product meets the design requirements. Learn verification and validation techniques for software quality assurance. Training is current with respect to regulations, procedures, and 3rd party validated and/or accredited. We know there is often a lack of training funds or time in your schedule to attend full days of training. We know there is often a lack of training funds or time in your schedule to attend full days of training. Understand how to ensure that software meets requirements and performs as expected. Software verification and validation (v&v) course by software excellence academy is designed to provide a knowledge base and practical skills for anyone interested in implementing or. Gain insights into fda regulations with fda software validation guidelines. This course covers the principles of software validation, computer systems validation and requirements for electronic records and electronic signatures. In just two weeks, you'll gain a comprehensive understanding of industry. Courses include voiceovers, easy navigation, reading materials, case. Course objectives include the following: Document your dedication to regulations, gxp compliance, quality, consumer safety, and job performance by earning a professional certification from biopharma institute. Fda software validation guidelines training. This course will help you understand software verification and validation and the impact of these new revisions on software used by medical device manufacturers. Fda software validation guidelines training by tonex. Learn verification and validation techniques for software quality assurance. Helping you become a certified csv. Software verification and validation (v&v) course by software excellence academy is designed to provide. Understand how to ensure that software meets requirements and performs as expected. Fda software validation guidelines training. Courses include voiceovers, easy navigation, reading materials, case. Helping you become a certified csv. Document your dedication to regulations, gxp compliance, quality, consumer safety, and job performance by earning a professional certification from biopharma institute. Training is current with respect to regulations, procedures, and 3rd party validated and/or accredited. Fda software validation guidelines training. Document your dedication to regulations, gxp compliance, quality, consumer safety, and job performance by earning a professional certification from biopharma institute. The “fundamentals of software testing & validation training” by tonex provides a comprehensive overview of essential software testing and validation. Helping you become a certified csv. This course covers the principles of software validation, computer systems validation and requirements for electronic records and electronic signatures. Fda software validation guidelines training. Courses include voiceovers, easy navigation, reading materials, case. Understand how to ensure that software meets requirements and performs as expected. Training is current with respect to regulations, procedures, and 3rd party validated and/or accredited. Trusted certificationaccredited online coursescpd / ceu accreditation Fda software validation guidelines training by tonex. Enhance your skills without leaving your office. We know there is often a lack of training funds or time in your schedule to attend full days of training. Software testing and validation are vital because they ensure that the end product meets the design requirements. This course will help you understand software verification and validation and the impact of these new revisions on software used by medical device manufacturers. Understand how to ensure that software meets requirements and performs as expected. Fda software validation guidelines training. Learn verification. Document your dedication to regulations, gxp compliance, quality, consumer safety, and job performance by earning a professional certification from biopharma institute. Software testing and validation are vital because they ensure that the end product meets the design requirements. Fda software validation guidelines training. Gain insights into fda regulations with fda software validation guidelines. Helping you become a certified csv. Training is current with respect to regulations, procedures, and 3rd party validated and/or accredited. This course covers the principles of software validation, computer systems validation and requirements for electronic records and electronic signatures. Helping you become a certified csv. Software testing and validation are vital because they ensure that the end product meets the design requirements. Trusted certificationaccredited online coursescpd. Fda software validation guidelines training. Learn verification and validation techniques for software quality assurance. Understand how to ensure that software meets requirements and performs as expected. This course will help you understand software verification and validation and the impact of these new revisions on software used by medical device manufacturers. Software verification and validation (v&v) course by software excellence academy. Gain insights into fda regulations with fda software validation guidelines. We know there is often a lack of training funds or time in your schedule to attend full days of training. The “fundamentals of software testing & validation training” by tonex provides a comprehensive overview of essential software testing and validation techniques. Courses include voiceovers, easy navigation, reading materials, case.. The “fundamentals of software testing & validation training” by tonex provides a comprehensive overview of essential software testing and validation techniques. Course objectives include the following: Software verification and validation (v&v) course by software excellence academy is designed to provide a knowledge base and practical skills for anyone interested in implementing or. Software testing and validation are vital because they ensure that the end product meets the design requirements. Trusted certificationaccredited online coursescpd / ceu accreditation Courses include voiceovers, easy navigation, reading materials, case. Training is current with respect to regulations, procedures, and 3rd party validated and/or accredited. Helping you become a certified csv. Fda software validation guidelines training by tonex. Learn verification and validation techniques for software quality assurance. Document your dedication to regulations, gxp compliance, quality, consumer safety, and job performance by earning a professional certification from biopharma institute. This course covers the principles of software validation, computer systems validation and requirements for electronic records and electronic signatures. Understand how to ensure that software meets requirements and performs as expected. Gain insights into fda regulations with fda software validation guidelines. This course will help you understand software verification and validation and the impact of these new revisions on software used by medical device manufacturers.Software Validation Online Training YouTube
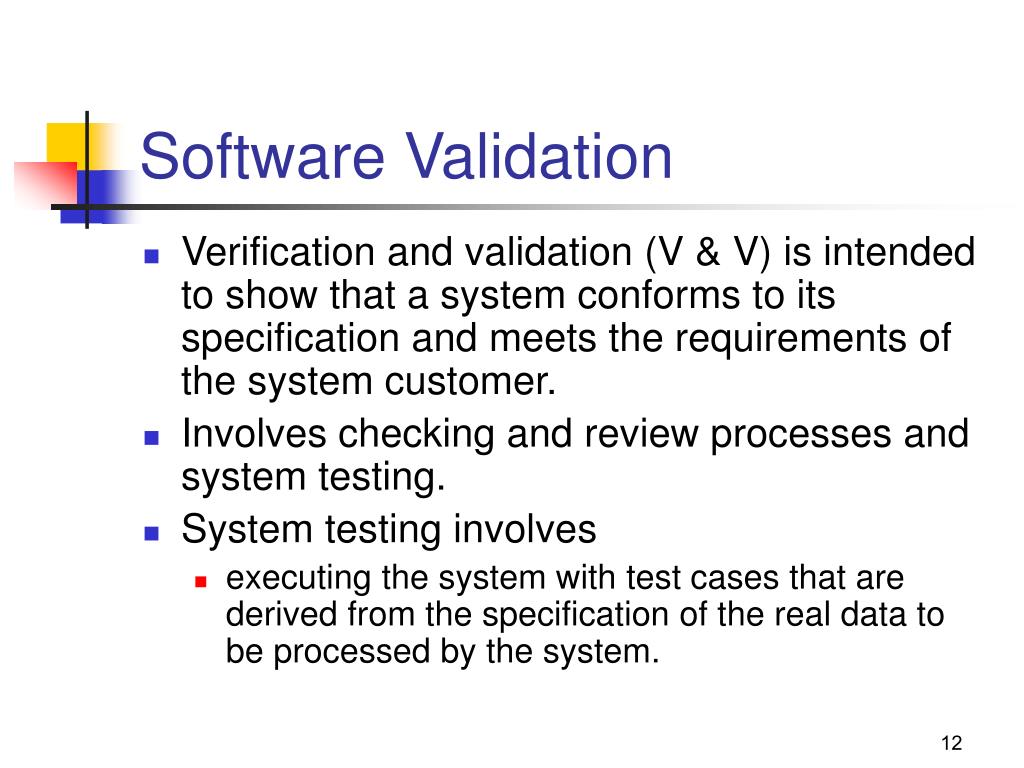
Software Verification, Validation and Compliance. V Shaped Model
What are the Software Validation Requirements of ISO 134852016
Software Validation Online Course And Certification
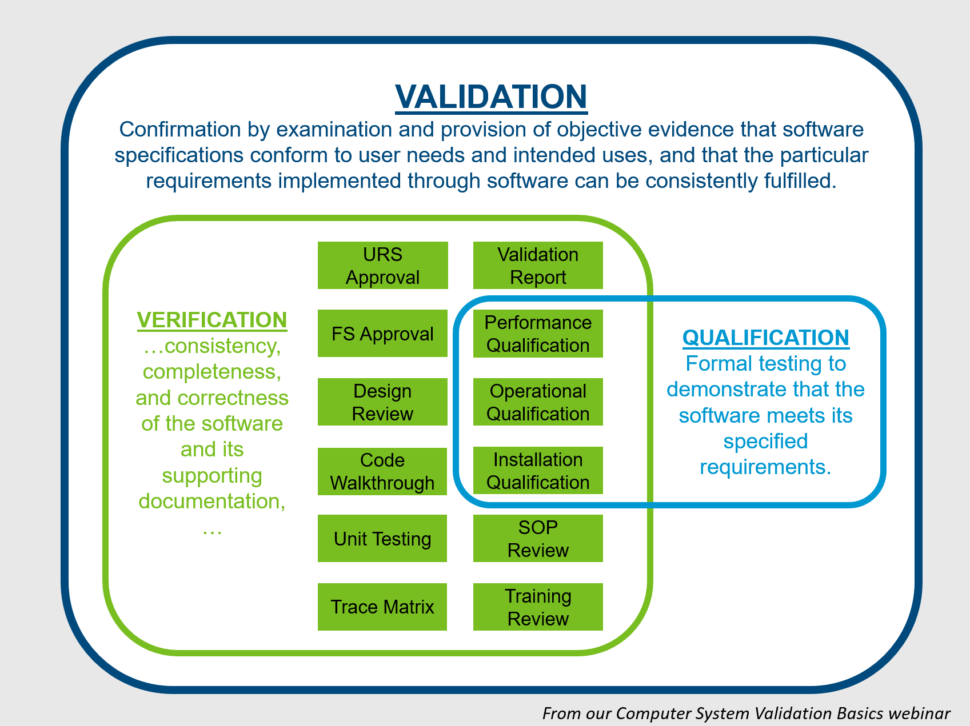
What is Computer System Validation and How Do You Do It?
Software Validation Online Course YouTube
PPT SWE 205 Introduction to Software Engineering PowerPoint
Software Validation (CSV) Online Course YouTube
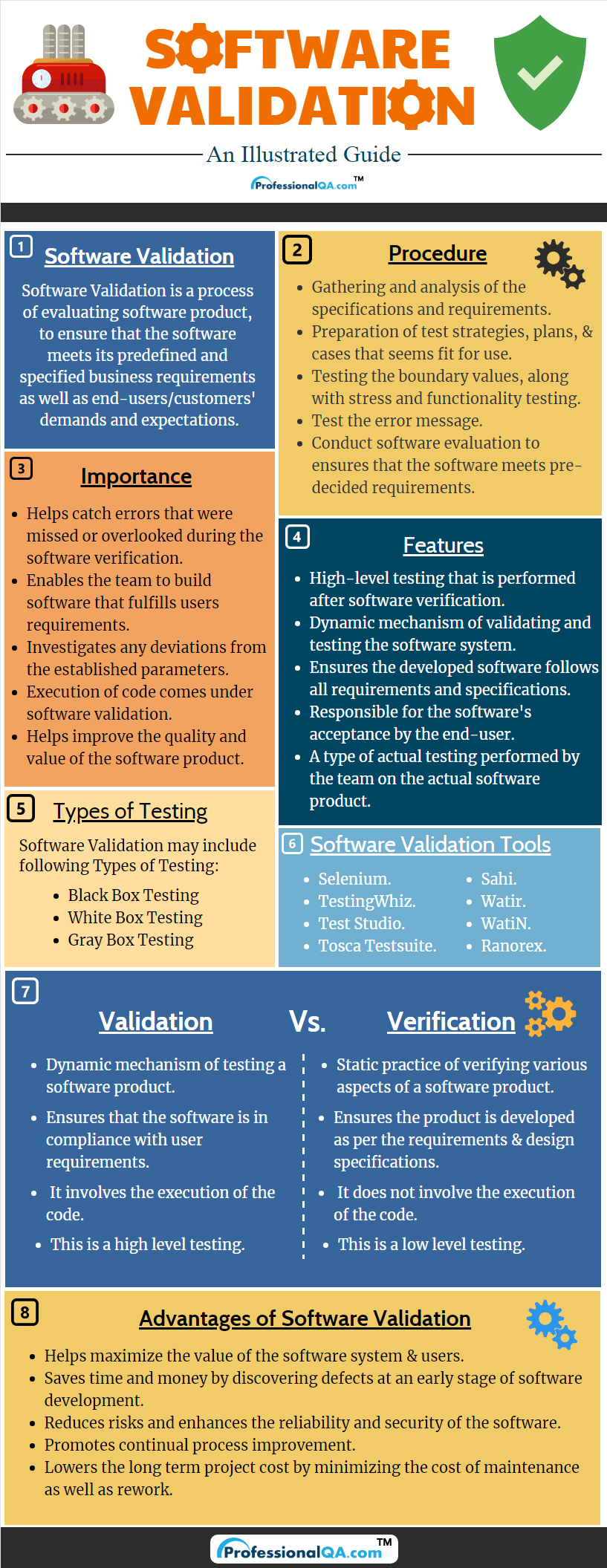
Software Validation Full Details PresentationEZE
Software Validation
Enhance Your Skills Without Leaving Your Office.
We Know There Is Often A Lack Of Training Funds Or Time In Your Schedule To Attend Full Days Of Training.
Fda Software Validation Guidelines Training.
In Just Two Weeks, You'll Gain A Comprehensive Understanding Of Industry.
Related Post: