Clinical Research Certificate Course
Clinical Research Certificate Course - The course will explain the basic principles for design of randomized clinical trials and how they should be reported. Transform you career with coursera's online clinical courses. Coursera offers a range of educational pathways in clinical research: Our course series on ich good clinical practice equips trial investigators and clinical researchers with the essential knowledge required to conduct reliable and ethical clinical trials. If you are simply interested in properly understanding the published literature or if you are embarking on conducting your own research, this course is your first step. Clinical trials courses cover a variety of topics essential for understanding and conducting clinical research. From anticipating and planning for protocol events to conducting systematic reviews to synthesize. In this course, you’ll learn about the more advanced elements of managing clinical trials. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. Professional certificates that can help you showcase your skills in clinical research settings. This course is designed to introduce you to the basic principles and practices of good clinical practice (gcp), which are essential for conducting clinical trials and ensuring the safety and. In this course, you’ll learn about the more advanced elements of managing clinical trials. If you are simply interested in properly understanding the published literature or if you are embarking on conducting your own research, this course is your first step. Professional certificates that can help you showcase your skills in clinical research settings. Clinical trials courses cover a variety of topics essential for understanding and conducting clinical research. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. Our course series on ich good clinical practice equips trial investigators and clinical researchers with the essential knowledge required to conduct reliable and ethical clinical trials. The course will explain the basic principles for design of randomized clinical trials and how they should be reported. In the first part of the course, students will be introduced to terminology. Coursera offers a range of educational pathways in clinical research: In this course, you’ll learn about the more advanced elements of managing clinical trials. Coursera offers a range of educational pathways in clinical research: From anticipating and planning for protocol events to conducting systematic reviews to synthesize. If you are simply interested in properly understanding the published literature or if you are embarking on conducting your own research, this course. Transform you career with coursera's online clinical courses. Clinical trials courses cover a variety of topics essential for understanding and conducting clinical research. In this course, you’ll learn about the more advanced elements of managing clinical trials. This course is designed to introduce you to the basic principles and practices of good clinical practice (gcp), which are essential for conducting. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. These include the basics of clinical trial design, regulatory requirements, and ethical. Transform you career with coursera's online clinical courses. In this course, you’ll learn about the more advanced elements of managing clinical trials. Clinical trials courses cover a variety. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. In this course, you’ll learn about the more advanced elements of managing clinical trials. Coursera offers a range of educational pathways in clinical research: Professional certificates that can help you showcase your skills in clinical research settings. If you are. Transform you career with coursera's online clinical courses. Coursera offers a range of educational pathways in clinical research: Clinical trials courses cover a variety of topics essential for understanding and conducting clinical research. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. This course is designed to introduce you. Professional certificates that can help you showcase your skills in clinical research settings. The course will explain the basic principles for design of randomized clinical trials and how they should be reported. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. Clinical trials courses cover a variety of topics. These include the basics of clinical trial design, regulatory requirements, and ethical. In this course, you’ll learn about the more advanced elements of managing clinical trials. The course will explain the basic principles for design of randomized clinical trials and how they should be reported. In the first part of the course, students will be introduced to terminology. Our course. These include the basics of clinical trial design, regulatory requirements, and ethical. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. Our course series on ich good clinical practice equips trial investigators and clinical researchers with the essential knowledge required to conduct reliable and ethical clinical trials. From anticipating. The course will explain the basic principles for design of randomized clinical trials and how they should be reported. Transform you career with coursera's online clinical courses. In the first part of the course, students will be introduced to terminology. Clinical trials courses cover a variety of topics essential for understanding and conducting clinical research. This course presents critical concepts. From anticipating and planning for protocol events to conducting systematic reviews to synthesize. In the first part of the course, students will be introduced to terminology. Coursera offers a range of educational pathways in clinical research: This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. Transform you career with. In the first part of the course, students will be introduced to terminology. This course presents critical concepts and practical methods to support planning, collection, storage, and dissemination of data in clinical research. The course will explain the basic principles for design of randomized clinical trials and how they should be reported. Our course series on ich good clinical practice equips trial investigators and clinical researchers with the essential knowledge required to conduct reliable and ethical clinical trials. Coursera offers a range of educational pathways in clinical research: Clinical trials courses cover a variety of topics essential for understanding and conducting clinical research. These include the basics of clinical trial design, regulatory requirements, and ethical. Transform you career with coursera's online clinical courses. If you are simply interested in properly understanding the published literature or if you are embarking on conducting your own research, this course is your first step. Professional certificates that can help you showcase your skills in clinical research settings.Home 1 Global Pharma
Certificate course in " Clincal Research & Clinical Data Management
Clinical Research Training Course I Clinical Research Online Training I
Clinical Research Certification Training. Graduate Training for
Best Clinical Research Certificate Courses in India
JLI Blog
why choose clinical research
Clinical Research Certification BioGrademy
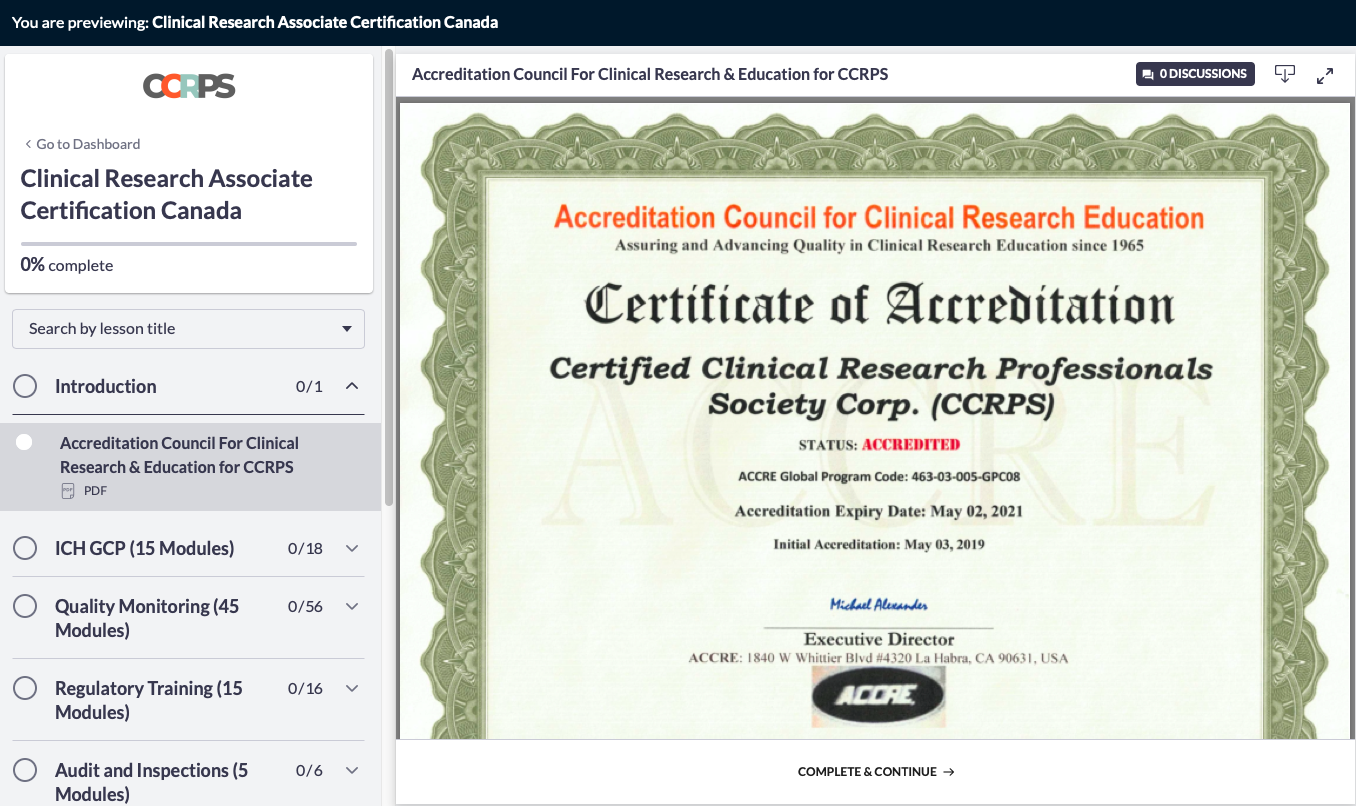
Clinical Research Associate Certification Canada Guide to A
3 months online certificate course in clinical research Global Pharma
In This Course, You’ll Learn About The More Advanced Elements Of Managing Clinical Trials.
This Course Is Designed To Introduce You To The Basic Principles And Practices Of Good Clinical Practice (Gcp), Which Are Essential For Conducting Clinical Trials And Ensuring The Safety And.
From Anticipating And Planning For Protocol Events To Conducting Systematic Reviews To Synthesize.
Related Post: